Treatment & Research News
Is Fibro an Autoimmune Disease?
Home | Treatment & Research News

Could something in your blood be causing fibromyalgia? Yes, says Andreas Goebel, M.D., Ph.D., of Liverpool, UK, along with his collaborators at King’s College London and the Karolinska Institute in Sweden.* The researchers injected mice with serum from fibromyalgia patients and within two days the rodents developed widespread pain. The mice also exhibited signs of fatigue and reduced muscle strength.
Why the symptoms develop is unclear but Goebel’s research points to the possibility that fibromyalgia is an autoimmune disease.
The immunoglobulin G (IgG) portion of the serum is loaded with antibodies and appears to be the pain-producing culprit. Mice injected with fibromyalgia serum devoid of the IgG fraction failed to cause symptoms. But could it be that human IgG makes mice pain-sensitive, regardless of the source (healthy controls or fibro patients)? No. Serum from healthy subjects didn’t produce symptoms in the mice.
Goebel suspects the IgG contains an autoreactive component that causes your many symptoms. But how can this be possible in the absence of tissue damage that normally exists in autoimmune diseases? Goebel says the antibodies can attack the sensory nerves or nearby cells to change their function (not destroy them). As a result, the nerves generate more pain signals traveling to the spinal cord.
Pain-Producing Antibodies
Examining the serum-injected mice revealed IgG antibodies clustered around special immune cells called satellite glial cells (SGCs). As shown in Figure 1, the SGCs surround the cell bodies of the sensory nerves. Antibodies on the surface of SGCs can amplify sensory signals just before they enter the spinal cord. In addition, antibodies in the IgG can increase the amount of distress chemicals secreted by the SGCs. Not only do these chemicals activate the sensory neurons, but they can also enter the cerebral spinal fluid to cause havoc in the central nervous system.
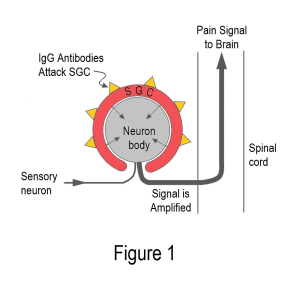
Widespread pain without tissue destruction is a credibility nightmare for fibromyalgia patients. Yet, Goebel simply injected fibromyalgia serum into mice to cause pain and other symptoms. This study certainly lays the groundwork for showing fibromyalgia is an autoimmune disease.
“Antibody-mediated immune processes in chronic primary pain (such as fibromyalgia) have been hiding in plain sight,” says Goebel. He adds that the antibody attack on the SGCs can’t be imaged, and lab tests cannot detect this process. Goebel’s findings also challenge the assumption that a person’s pain level corresponds to the degree of visible tissue destruction.
Injecting serum from patients into rodents is called a passive transfer study. It’s only been done in a few other diseases. Although the current project involved patients from two different centers, the findings need to be replicated.
One last point: people are not mice. How do the researchers know the SGCs are the cells under attack in humans? Goebel’s colleagues incubated the antibodies from fibromyalgia patients and healthy controls with SGCs taken from seven post-mortem subjects (none had fibromyalgia). Using a fluorescent dye and electron microscopy, only the antibodies from fibromyalgia patients heavily coated the SGCs. It’s as though the fibromyalgia antibodies are drawn to the SGCs like iron to a magnet.
Game Changer for Fibromyalgia
If Goebel’s work stands the test of time, fibromyalgia will be an autoantibody type of pain. This could be a game changer for fibromyalgia research because the condition is currently viewed as a dysfunctional central nervous system. Admittedly, the brain and spinal cord do not operate properly, but the cause remains unknown. However, if antibodies are disrupting the SGCs, this could be the autoimmune trigger that causes the nervous system commotion.
Research points to multiple abnormalities in the central nervous system. The spinal cord contains chemical imbalances. The pain control system doesn’t work, and the brain centers fail to contain the barrage of pain signals. Sleep is disrupted, hormones are dysregulated, and cognitive functions are diminished.
The foregoing findings are often packaged into the central sensitization theory to explain pain without a triggering source. It assumes that the central nervous system is hypersensitized to incoming sensory signals, but no one knows why. This, in turn, leads to an abnormally exaggerated response. In the case of fibromyalgia, a harmless trickle of nerve impulses is transformed into widespread pain and other symptoms.
“Some say you don’t need a driving source to sustain central sensitization,” says Goebel, “but it has never been shown convincingly in any animal model. That’s why many of us (physicians and researchers) never really believed it.” Based on the passive transfer study, it’s more plausible that fibromyalgia is an autoimmune disease.
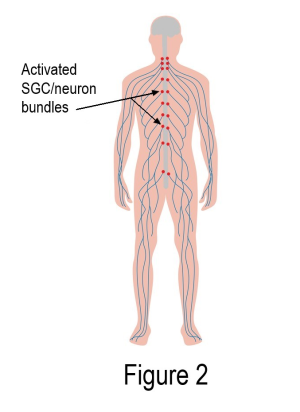
IgG with autoreactive antibodies clustered around the SGCs may be the missing piece to the fibromyalgia puzzle. As shown in Figure 2, activated SGCs form a pain-generating circuitry up and down both sides of the spinal cord (each red dot represents thousands of SGC/neuron units). Hurting from head to toe would be expected, not questioned! The spinal cord and brain would naturally be thrown into turmoil.
Study Implications
Wondering why your body is generating antibodies that adhere to the SGCs? Examining the mice won’t answer this question. “Our model takes it from the point where the IgG antibodies are already produced,” says Goebel. However, dissecting the tiny fraction of the IgG that is pathogenic (autoreactive) could lead to a disease marker. Goebel’s colleagues in Sweden are working on this.
Your B cells make the antibodies in your IgG. If your antibodies are misbehaving, it is caused by a problem with your B cells. Your antibodies are just doing what your B cells programmed them to do. It’s your B cells that need a closer look and fortunately, this is part of a recently approved AFSA study.
What about treatments? The fibromyalgia-like symptoms go away once the IgG antibodies work their way out of the mouse’s body. So, approaches that dislodge the IgG antibodies from the SGCs should work, even if they do not stop the antibody production.
Currently, therapies in this category are extremely expensive and not yet available to fibromyalgia patients. However, a small “proof-of-concept” type of trial is underway to test an intravenous drug called rozanolixizumab.
Alternatively, medications that reduce SGC activation may help. For example, low-dose naltrexone targets specific receptors on the SGCs to quiet them down. While removing the harmful antibodies from the SGCs is more effective, this approach is available and cheap.
Presuming autoantibodies to your SGCs are driving your symptoms, this may explain why medications that work in the spinal cord produce dismal results. Examples include the three FDA-approved drugs (pregabalin, duloxetine, and Savella) that operate downstream of the SGCs. Using them could be the equivalent to putting a bucket under a leaky faucet, while targeting the SGCs would be more akin to repairing the faucet.
Bottom Line
Fibromyalgia will gain more credibility once Goebel’s work is replicated and the word spreads about the passive transfer study. Many physicians might remain skeptical about the cause of your symptoms, especially given that roughly half view fibro as psychologically induced. However, over time, doctors might be willing to consider that fibromyalgia is an autoimmune disease. Admittedly, it is not the classical type of autoimmunity that causes tissue destruction but rather changes in cellular function.
In the meantime, Goebel’s mouse model is still useful for fibromyalgia research. Examining serum-injected mice can highlight what the SGCs are doing to cause symptoms. In addition, the mouse model may assist with developing possible treatments. It could be a long road ahead, but at least research will be moving in the right direction.
Stay Current on Treatments & Research News: Sign up for a Free Membership.
Symptoms | Medications | Alternative Therapies | Muscle Pain Relief | Fibro Friendly Exercises
* Goebel A, Krock E, Gentry C, et al., 2021. Passive transfer of fibromyalgia symptoms from patients to mice. J Clin Invest. 131(13):e144201. Free Report
