Projects Funded
Low-Dose Naltrexone (LDN) for Treating Fibro
Principal Investigators: Jarred Younger, Ph.D., and Sean Mackey, M.D., Ph.D.
Stanford University, Palo Alto, California
(2008)
Relief from chronic pain is difficult to achieve, and this may be partly explained by the fact that there are two targets: the neurons and the nearby microglial cells. All drugs to date were designed to work on the neurons, but some of them can alter the function of the microglia. Naltrexone is one of them.
The microglia are immune cells tasked with protecting your central nervous system (CNS) against any type of threat, such as viruses, that could harm the brain. When it comes to pain transmission, these cells usually sit quietly on the sidelines. However, many substances that can activate these immune cells are elevated in the spinal fluid of people with fibromyalgia.
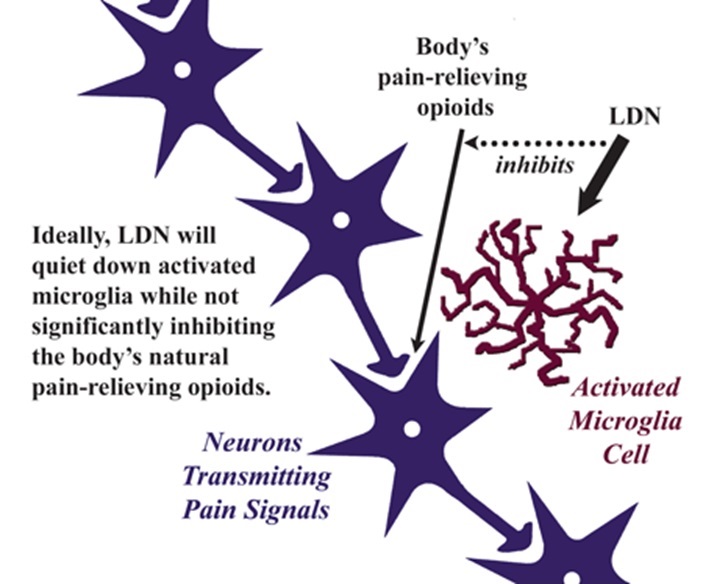
What happens when the microglia are in their activated state? They pour out cytokines and other chemicals that cause flu-like symptoms, such as widespread achiness, sleep disruption, profound fatigue, concentration difficulties and depressed mood. Anyone who has had a bad case of the flu knows what it feels like when their microglia are raising a ruckus. At this point, the person may have mild inflammation that may not show up on routine lab tests. Ordinarily the microglia settle down after a few days and the symptoms disappear.
Younger and Mackey propose that the microglia are stuck in a chronically activated or inflammation-promoting state in fibromyalgia and will test the ability of low-dose naltrexone (LDN) to relieve the symptoms of this condition. This represents the first treatment trial in fibromyalgia that is designed to target the microglia instead of the neurons.
Naltrexone is available in 50 mg pills to treat opioid drug cravings in addicts. At this dose, the drug blocks pain-relieving chemicals such as endorphins (the body’s own opioids). But, even at low doses, the drug also blocks the activation of the microglia so they do not produce pain-promoting substances. The key is to use a small dose of naltrexone (3-4.5 mg), which may still “chill out” the microglia while not appreciably interfering with the body’s own opioid pain relievers.
The ability of naltrexone to shut down the pain and other symptom-generating activities of the microglia appears to be safe with very few side effects. It has been tested in small trials for the treatment of Crohn’s disease and multiple sclerosis pain. Now it will be determined if the drug can benefit individuals with fibromyalgia.
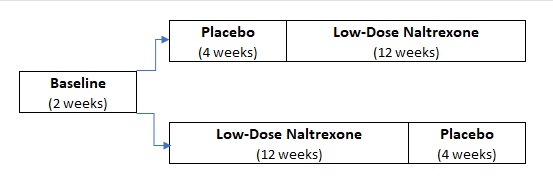
Thirty people with fibromyalgia will participate in the trial. For part of the study (12 weeks), these individuals will receive low-dose naltrexone (LDN) every night before bedtime. For another part of the study, they will receive an inactive placebo. Neither the participants nor the researchers will know what the participants are receiving until the study is completed. Participants will start on either the drug or placebo and will then switch to the other substance.
Participants will fill out a short questionnaire every night to chart their pain levels, sleep quality, physical activity, mood, and fatigue. These symptom scores will be collected on hand-held computers to test the drug’s effectiveness.
Trial Results Promising
“Three out of ten people trying LDN will reap a significant improvement in pain, as well as fatigue or sleep,” says Jarred Younger, Ph.D. “This is twice as high as the current FDA-approved treatments for fibromyalgia, which typically provide a 15 percent response rate.” The study results are published in the February 2013 issue of Arthritis & Rheumatism.1
What does “significant improvement” really mean? Younger’s team required LDN responders to show at least a 30 percent reduction in pain PLUS a 30 percent improvement in either fatigue or sleep. Patients also showed benefits in general satisfaction with life and mood.
The overall impact of a drug on people with multi-symptom conditions (like fibromyalgia) is often measured as Patient Global Impression of Change. At the end of the treatment trial, Younger states, “Half of the participants reported feeling ‘much improved’ or ‘very much improved’ from taking LDN.” 2
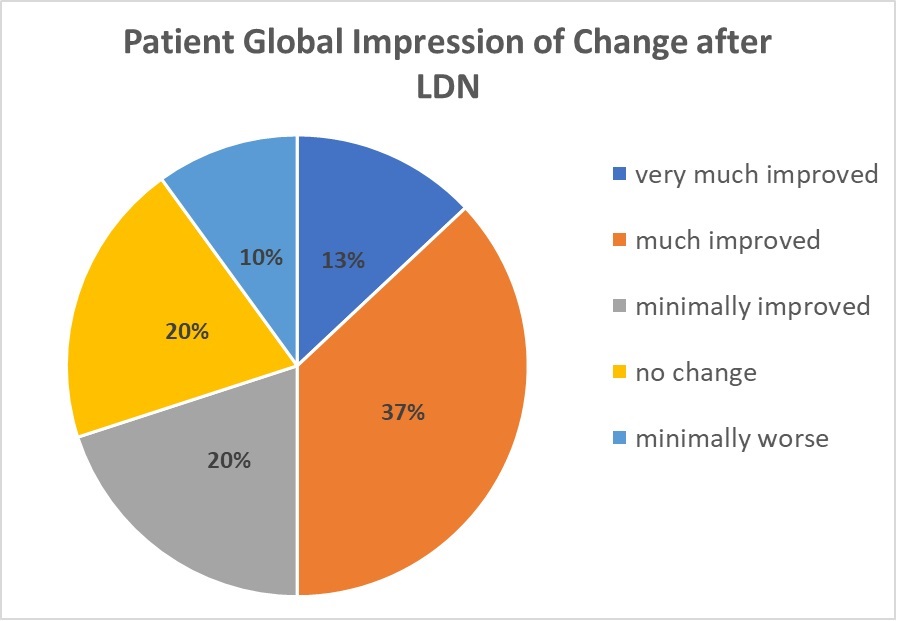
It would be impossible for any drug to provide the overall benefits shown in the chart without reducing the energy-zapping fatigue associated with fibromyalgia. “Even though over 30 percent of the participants had a strong improvement in fatigue,” says Younger, “the group average was not significant.” Rather than looking at group averages from the study, responder rates showed LDN relieved fatigue in three out of ten patients.
Pain severity or duration of symptoms didn’t predict who benefited from the drug in this study. The same findings were found by the same research team in a prior trial of ten fibromyalgia patients (also partially funded by AFSA).3
LDN doesn’t work overnight. Symptom improvements from this medication took at least a month to show up … and for many patients it can be up to three months.
Probably the greatest advantage LDN has over most drugs prescribed for fibromyalgia is its very low incidence of side effects. Patients didn’t know if they were taking LDN or the placebo, yet they rated them equally tolerable. The only two side effects that occurred more frequently when taking LDN were vivid dreams and headaches.
Vivid dreams were described as being more colorful and rememberable. Any person experiencing an unpleasant side effect was put on a lower dose of 3 mg before bedtime (as opposed to 4.5 mg). This did the trick because no one dropped out of the study.
Although LDN is relatively cheap ($30/month or less), this medication has one major drawback: the low-dose version must be prepared at a compounding pharmacy (as an immediate-release formula), which means insurance companies will not pick up the tab. In addition, naltrexone (even at low doses) may interfere with the pain-relieving action of opioid medications. Patients taking opioids may require a lower starting dose.
AFSA’s LDN Trial Snapshot
- 3 ut of 10 patients significantly improved
- takes at least one month to reap the benefits
- low side effects – vivid dreams and headaches
- must be compounded for low dose; insurance doesn’t cover
- targets microglia function rather than the neurons, so it may augment other treatments designed to improve neuron function
- Younger J, Mackey S, et al. Low-Dose Naltrexone for the Treatment of Fibromyalgia. Arthritis Rheum 2013; 65(2):529-538. The report is free to read but not print.
- Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol 2014; 33:451-459. It’s free to print.
- Younger J, Mackey S. Fibromyalgia Symptoms Are Reduced by Low-Dose Naltrexone: A Pilot Study. Pain Med 2009; 10(4)633-72. It’s free to print.
A Decade of LDN Experience
After the AFSA-funded trial on LDN was published in 2013, additional studies involving this drug have appeared in the medical journals. Key findings are highlighted below. In addition, if you are interested in trying LDN, see our Medications section. Several physicians offer practical advice in our article: Giving LDN Your Best Shot.
Brain Microglia Activation Found – Three brain imaging studies, including one funded by AFSA, have shown that the microglia in fibromyalgia patients are activated compared to healthy controls.1,2,3 These reports help support the rationale for using LDN because this drug is known to reduce the activation of these cells. For details about the project funded by AFSA, click here.
Pro-Inflammatory Cytokines Reduced by LDN – Upon activation, microglia can signal the peripheral tissues to secrete more cytokines, which end up in the bloodstream. Blood assays before and after an eight-week trial of LDN in people with fibromyalgia found that the drug significantly reduced the level of 17 cytokines.4 So, not only are the microglia activated in fibromyalgia, but they may also be causing a low-level type of inflammation in the tissues that is not responsive to aspirin or NSAIDs.
No Serious Side Effects – A literature review involving over 11,000 people taking naltrexone (daily dose ranging from 3 to 320 mg) revealed no harmful adverse events over that of placebo.5 However, LDN can still produce mild side effects that occur infrequently. Aside from vivid dreams and headaches reported in the AFSA trial, patients may experience nausea, diarrhea, anxiety, and transient insomnia.6
LDN Might Reduce Opioid Use – Analysis of 15,000 patients who began using LDN after a documentary on the treatment aired in Norway showed that opioid consumption went down 46 percent.7 If this drop in opioid use had been due to concerns that LDN might interfere with the action of opioids, then an increase in other classes of medications would have been expected. However, the use of alternative pain-relievers did not go up.
- Albretch DS, et al. Brain glial activation in fibromyalgia – a multi-site positron emission tomography investigation. Brain Behav Immun 2019; 75:72-83. Journal Report
- Seo S, at al. Abnormal neuroinflammation in fibromyalgia and CRPS using [11C]-(R)-PK11195 PET. PLoS One 2021; 16(2):e0245152. Journal Report
- Mueller C, Younger JW, et al. Evidence of neuroinflammation in fibromyalgia syndrome: a [18F]DPA-714 position emission tomography study. PAIN 2023 Oct 1; 164(10):2285-2295. AFSA-funded Study Article
- Parkitny L, Younger J. Reduced Pro-Inflammatory Cytokines after Eight Weeks of Low-Dose Natrexone for Fibromyalgia. Biomedicines 2017; 5(2):16. Journal Report
- Bolton M, et al. Serious adverse events reported in placebo randomized controlled trials of oral naltrexone: a systemic review and meta-analysis. BMC Med 2019; 17(1):10. Journal Report
- Driver CN, D’Souza RS. Efficacy of Low-Dose Naltrexone and Predictors of Treatment Success or Discontinuation in Fibromyalgia and Other Chronic Pain Conditions: A Fourteen-Year Enterprise-Wide Retrospective Analysis. Biomedicines 2023; 11(4):1087. Journal Report
- Raknes G, Smabrekke L. Low-dose naltrexone and opioid consumption: a drug utilization cohort study based on data from the Norwegian prescription database. Pharmacoepidremiol Drug Saf 2017; 26(6):685-693. Journal Report
